Our bodies are home to trillions of microbes. They help us digest food, balance our immune system, and keep our hearts and brains healthy. But harmful viruses and bad bacteria can creep in at any time. Scientists at La Jolla Institute for Immunology are working to stop these infectious agents and learn more about the body’s defense strategies.
At first glance, dengue virus looks a bit like a kid’s toy. The virus is shaped like a little ball, and the pieces of its outer shell fit together like puzzle pieces. But as soon as it invades, pieces of dengue’s smooth shell pop open to release dengue’s genetic material into our cells.
This sneak attack is extremely effective. According to the World Health Organization, there are an estimated 390 million dengue infections each year, and the disease is a leading cause of serious illness and death in some Asian and Latin American countries.
In fact, mosquito-borne diseases like dengue, malaria, and Zika are spreading around the globe as warmer temperatures allow mosquito species to migrate out of their usual habitats. Overall, many infectious diseases are reaching new territories. Scientists used to think Ebola virus was only a threat in Central Africa—until the virus crossed international borders and exploded into a 30,000-person epidemic.
Other microbes hide in plain sight. An estimated 50 percent of San Diegans harbor cytomegalovirus deep in their tissues. The virus will go unnoticed by most people, but when it infects pregnant women, it can cause serious birth defects. In fact, it is the most common infectious cause of birth defects in the United States.
We still need to know much more about infectious diseases and how to effectively treat, or better yet, prevent them. That’s why several teams of LJI researchers have dedicated their careers to understanding and defeating dangerous microbes.
The rapid killers
Endemic in most of West Africa, Lassa fever tends to start out with a slight fever and general malaise like any old cold or flu. It doesn’t seem like an emergency at first and for the majority of infected people it is indeed nothing more than a blip of feeling unwell for a bit. But in 20 percent of patients, the disease progresses to full blown hemorrhagic fever, which can result in multi-organ failure and death. In West Africa, the virus causes 300,000 to 500,000 illnesses annually and kills thousands each year.
LJI Professor Erica Ollmann Saphire, Ph.D., is fascinated by the simplicity of Lassa virus and other hemorrhagic fever viruses, such as Ebola and Marburg. Lassa virus carries out a sophisticated attack using just four genes and four moving parts. Ebola virus has just seven genes and eight moving parts. “How can something so simple be so spectacularly pathogenic?” says Dr. Saphire. “On the other hand, with so few moving parts, we can find where it is vulnerable and launch a therapeutic.”
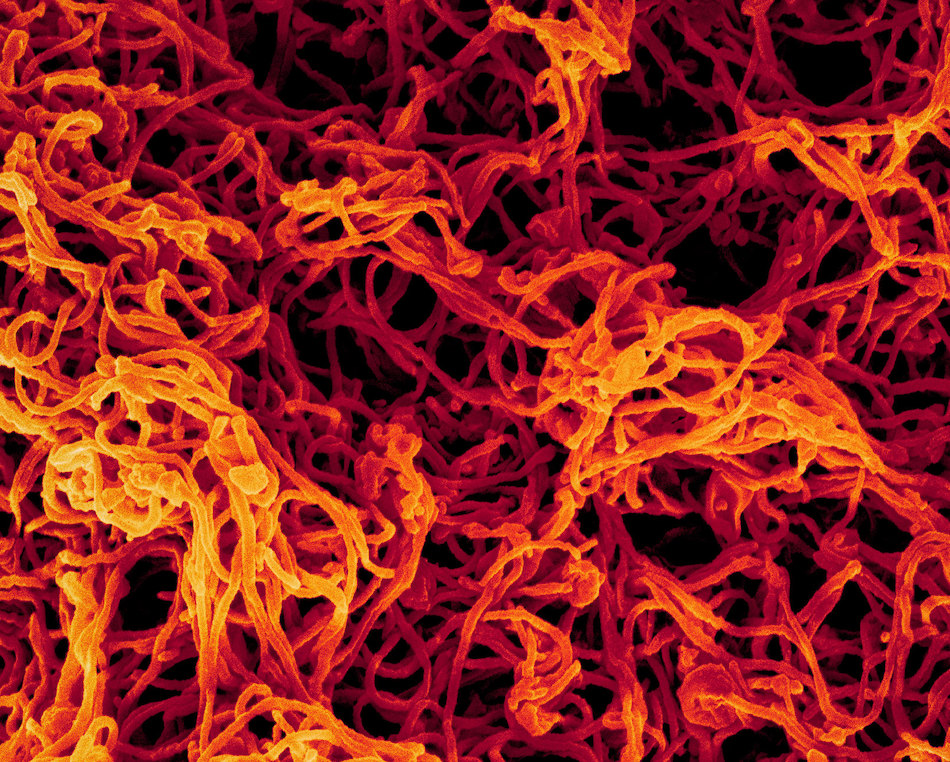
New cryo-electron microscopy imaging equipment at LJI makes it possible for Dr. Saphire and her colleagues to quickly see the smallest details of viral structures and where these pathogens are susceptible to human antibodies and drug therapies.
Another one of LJI’s strengths is its connections with experts in countries where many infectious diseases are endemic. Dr. Saphire is currently working alongside epidemiologists and medical professionals in Sierra Leone and the Democratic Republic of Congo. At the same time, LJI Associate Professor Sujan Shresta, Ph.D., is coordinating with experts in Nepal to study mosquito-borne diseases in that country. “We need their expertise and local knowledge,” says Dr. Shresta.
Dr. Shresta, who was born and raised in Nepal before she moved to the United States to continue her higher education, wants to help people in both regions. She’s seen how diseases such as dengue devastate communities in Nepal, and she says new mosquito-borne diseases could easily reach LJI’s home in Southern California. “The same mosquitoes that transmit dengue can be found right here in San Diego,” she says, “and to be prepared, we need to learn more about these diseases before they eventually reach the U.S.”
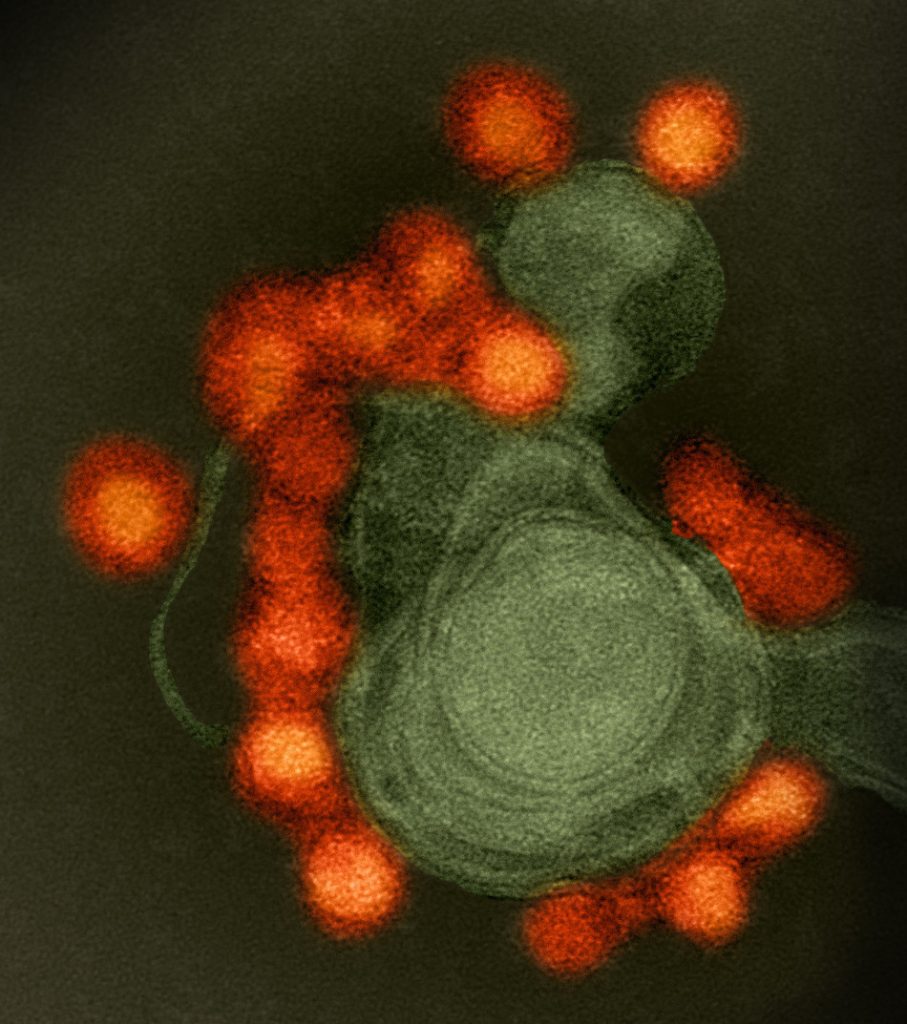
Dr. Shresta focuses on how viruses interact with the host immune system. She says the closely related dengue and Zika viruses show how complicated the host response can be. Studies suggest that when a person is infected with dengue, their response to a later Zika infection will be much worse and vice versa. This “cross-reactivity” poses a problem for both dengue and Zika vaccine programs, where priming the body to fight one virus may leave it vulnerable to another closely
related virus.
“This is really important to study because I believe the most effective way to solve the global dengue/Zika problem is going to be a vaccine, and we’re showing that you can’t approach vaccine development focusing on only one virus at a time.” Dr. Shresta says.
The turncoats
We’re all home to viruses and bacteria. Some protect you or even help you digest food. And then there are the double agents, the microbes that are usually harmless, until the day they decide to attack. What makes these pathogens turn on us?
Take group A Streptococcus, a noodle-shaped bacterium that makes up a normal part of the body’s microbiome—except when it turns on the body, triggering tonsillitis, which is commonly known as strep throat. Just about every kid gets strep throat at some point. But some kids get it over and over again, and it’s more dangerous than you may think. Left untreated, strep throat can actually lead to rheumatic heart disease.
LJI scientists are working to figure out why. “It’s still unknown why children get recurrent group A strep,” says Jennifer Dan, M.D., Ph.D.
As a member of Professor Shane Crotty’s lab at LJI (she also has an assistant professor appointment at the University of California, San Diego), Dr. Dan studies exactly how the body responds to group A strep. This project could provide insight into how we fight off many other infectious diseases.
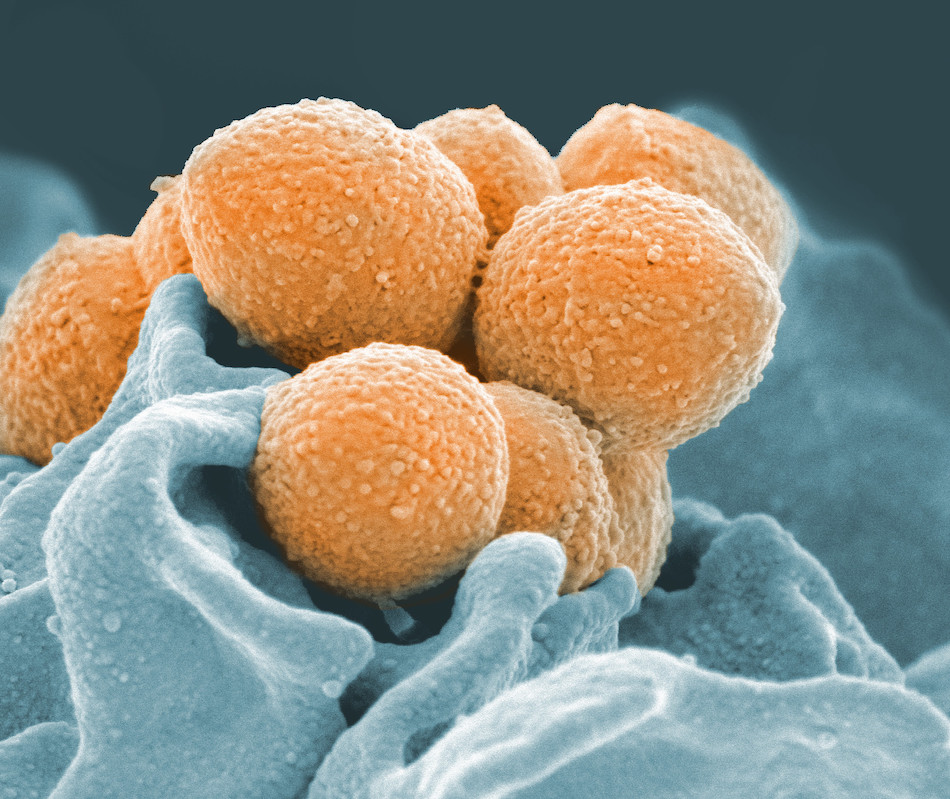
Mitchell Kronenberg, Ph.D., LJI President and Chief Scientific Officer, says work on diseases like group A strep can help us understand how the body responds to a huge range of attacks. “The principles we learn can be applied across infections,” he says.
Dr. Kronenberg’s own lab is very interested in another infectious turn-coat: Streptococcus pneumoniae. This bacterium can live harmlessly in the body but then turn around and cause disease in immunocompromised individuals, such as babies and the elderly. It is a leading cause of fatal pneumonia in older people. “These bacteria live on the nutrients from mucus: sugar, fats, and proteins. They normally stay away from immune cells,” says Dr. Kronenberg. “The real question is how does it decide to become invasive?”
Dr. Kronenberg says the Institute’s expertise in the techniques of flow cytometry and genome sequencing is giving his team new insights into how better vaccines against S. pneumoniae might move forward.
The standing armies
A tuberculosis infection can begin with a single sneeze from an infected person. With one sneeze, 40,000 droplets shoot into the air, and each droplet is loaded with Mycobacterium tuberculosis. You’d only need to inhale ten of these bacteria to contract the infection. And then the invaders lay low.
Tuberculosis is one of those diseases that mostly hangs out in a “latent” stage. The bacteria don’t actually want to kill their host, and yet they can become deadly when the immune system stops working as it should.
“Tuberculosis is currently the number-one killer worldwide in terms of infectious diseases,” says LJI Professor Bjoern Peters, Ph.D.
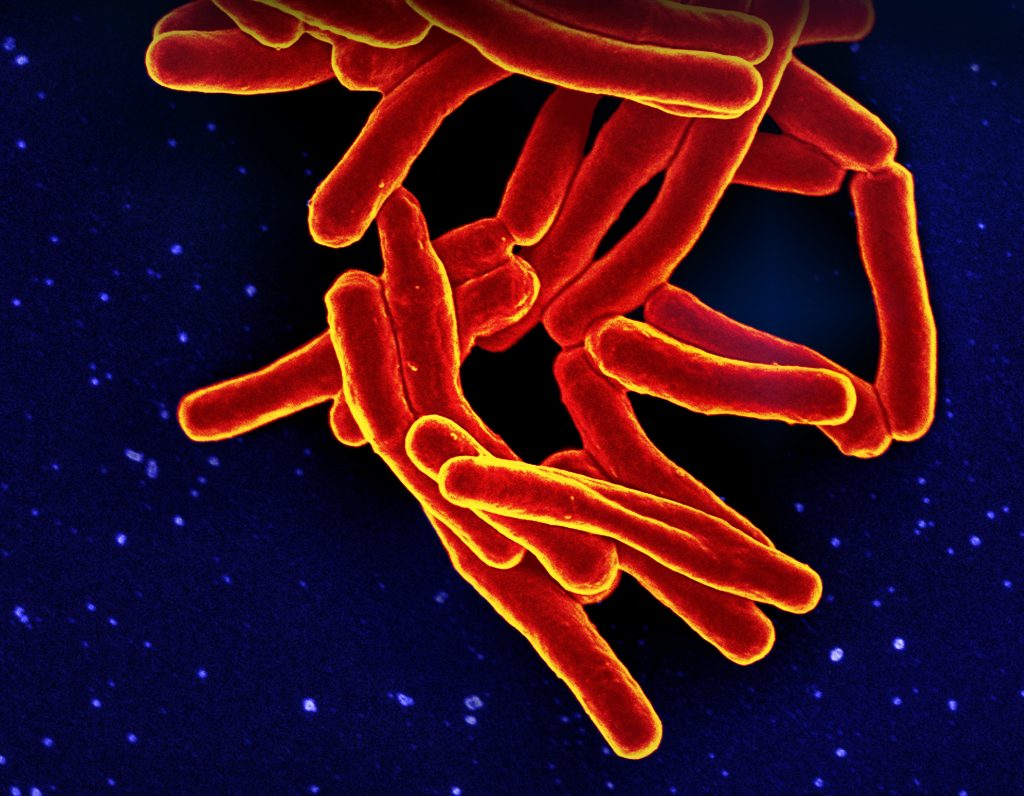
Dr. Peters and his team are working closely with the lab of LJI Professor Alessandro Sette, Dr. Biol.Sci., to answer a big question in tuberculosis disease research: Why do some infected people get sick when so many don’t show symptoms?
LJI researchers are searching for ways to catch tuberculosis cases before they turn deadly. Current therapies for tuberculosis tend to be harsh on the body, so they aren’t given proactively in most countries. Dr. Peters wants to find a way to treat those most at risk—sooner.
“We want to identify the immune system signals of someone who is about to get sick,” says Dr. Peters.
Drs. Peters and Sette have also established the Immune Epitope Database (IEDB), the largest existing collection of molecular targets or epitopes recognized by the immune system and an invaluable resource for infectious disease research around the world. The free database houses information on how immune cells such as T and B cells target pathogens.
“We can use these epitopes as bait to fish out the T cells that drive immune response and understand, for example, the type of immune response in people with good immune responses versus people whose immune response fails,” says Dr. Sette.
Dr. Sette’s work has also shed light on infectious diseases such as pertussis (whooping cough), Zika, dengue, malaria, chikungunya, cytomegalovirus (CMV), and shingles. The virus that causes shingles, called the varicellazoster virus (VZV), is another one of those pathogens with a long latent period.
If you’ve had chicken pox, then you now have VZV hiding out in your nerve cells. “The virus is still in a bit of a battle with your immune system, but it’s not really obvious that you’re infected,” says LJI Professor Chris Benedict, Ph.D. He says that shingles tends to be associated with elderly people because the virus becomes active again as the immune system weakens with age, but shingles also can occur in younger individuals, especially when they are stressed.
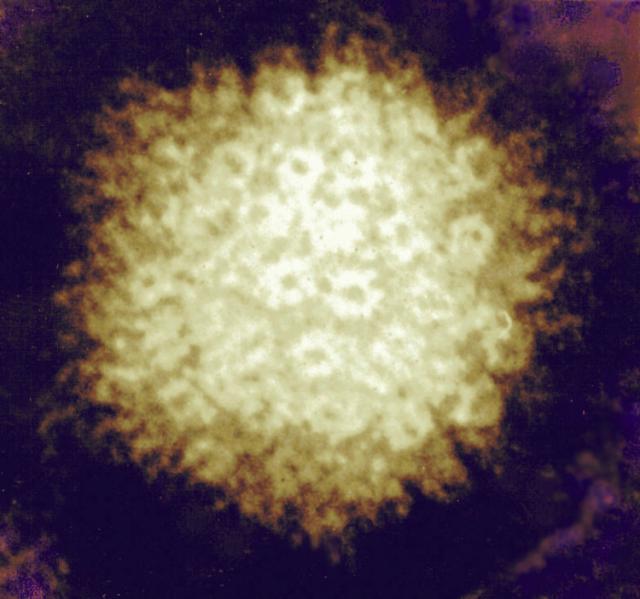
Dr. Benedict is also concerned with CMV, a common virus that can infect almost anyone.
The immune system of healthy people can keep the virus in check but can be very harmful to babies when their mother is infected during pregnancy. In adults with compromised immunity, especially due to organ transplantation, it can be fatal. A recent partnership with the Sette lab seeks to understand how immune “helper” cells called CD4 T cells respond specifically to CMV.
Dr. Sette says LJI has the right team of scientists and bioinformatics experts in place to figure out how mysterious latent infections turn deadly. “We have fantastic faculty and scientists who collaborate in the studies and really help extract every piece of information possible from the data,” he says.
The jack-of-all-trades
Some pathogens fit neatly into a category. And then there’s HIV. It can kill a person within a few years, or it can hide out in the body for a decade or more. It adapts quickly and blocks the immune system from even keeping an eye on it.
“This virus is especially difficult to target,” says J.H. Lee, Ph.D., a postdoctoral researcher in the Crotty lab. Dr. Lee says the immune system has trouble fighting off HIV because the virus is covered in a shield of sugar molecules called glycans. “It’s like it’s wearing an invisibility cloak,” Dr. Lee says.
But there could be a way to outsmart HIV. The Crotty Lab is part of an international consortium to develop an HIV vaccine. The group studies blood samples from some of the rare people who produce antibodies that are able to inactivate or neutralize the many strains of the virus. These unusual antibodies have given scientists a guide for what kinds of immune molecules an HIV vaccine would need to elicit in a person’s body. The Crotty lab is now leading research in animal models to study how an effective vaccine could work. At the same time, they are also studying healthy people to determine whether the general population has the potential to make those rare antibody-producing cells in response to a vaccine.
LJI’s state-of-the-art facilities and expertise make it the perfect place to spearhead this effort. “Because we have advanced molecular techniques here, we can do work on highly virulent pathogens like HIV or others, for example, using molecular pieces rather than the entire disease-causing organism. Therefore we can work safely without the need for super high levels of containment or fear that our scientists will be infected,” says Dr. Kronenberg.
With the help of Institute supporters and international collaborators, LJI researchers are closing in on the best ways to defeat the world’s worst pathogens.