Lupus knows no bounds. In most autoimmune diseases, inflammatory immune cells attack a specific organ—and one organ only. For example, in type 1 diabetes, T cells start killing cells in the pancreas.
But lupus is a “systemic” autoimmune disease, which means it prompts harmful immune cells to cause widespread damage. Lupus can strike anywhere—the joints, kidneys, skin, brain, and more. Lupus can even harm a person’s reproductive organs and increase the risk of miscarriage during pregnancy.
LJI Instructor Carolyn Moderbacher, Ph.D., is taking advantage of lupus’s systemic nature. She’s working with lupus patients to hunt down key immune cells in their nasal passages. By swabbing tissue deep within the nose, Dr. Moderbacher hopes to uncover exactly how immune cells drive this mysterious disease.
Dr. Moderbacher’s pioneering approach recently won her a Lupus Innovation Award and nearly $450,000 in funding from the Lupus Research Alliance, the world’s largest private funder of lupus research. “This research could be transformative, and it’s really exciting to have the funding to move it forward,” says Dr. Moderbacher, who works in the lab of LJI Professor and Chief Scientific Officer Shane Crotty, Ph.D.
In this Q&A, Dr. Moderbacher reveals how her work may uncover a new side of autoimmune disease.
Question: The Crotty Lab usually focuses on immunity to infectious diseases. How did you end up studying autoimmunity in lupus?
Dr. Moderbacher: Autoimmunity is sort of the other side of the same coin. Instead of trying to get a better immune response to vaccines, to treat autoimmune disease you’re trying to dampen an overexuberant, and misdirected, immune response.
I’ve always been interested in lupus because it’s an extremely complex disease where we have no targeted therapies or approaches that work in the majority of patients. I wanted to see if we could take some of the techniques that we had developed in the Crotty Lab— specifically for studying SARS-CoV-2 vaccine responses—and apply them in the context of autoimmunity to answer some important questions about lupus-specific B and T cells.
What’s the connection between the nasal passages and lupus?
A: The thing with lupus is that the disease is mediated by self-reactive antibodies, or “autoantibodies.” We know these autoantibodies develop in lymphoid tissue, but lymphoid tissues are not very well characterized in lupus patients, largely because it is difficult to access and repeatedly sample these sites.
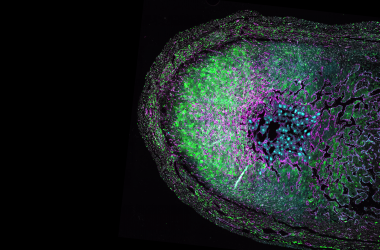
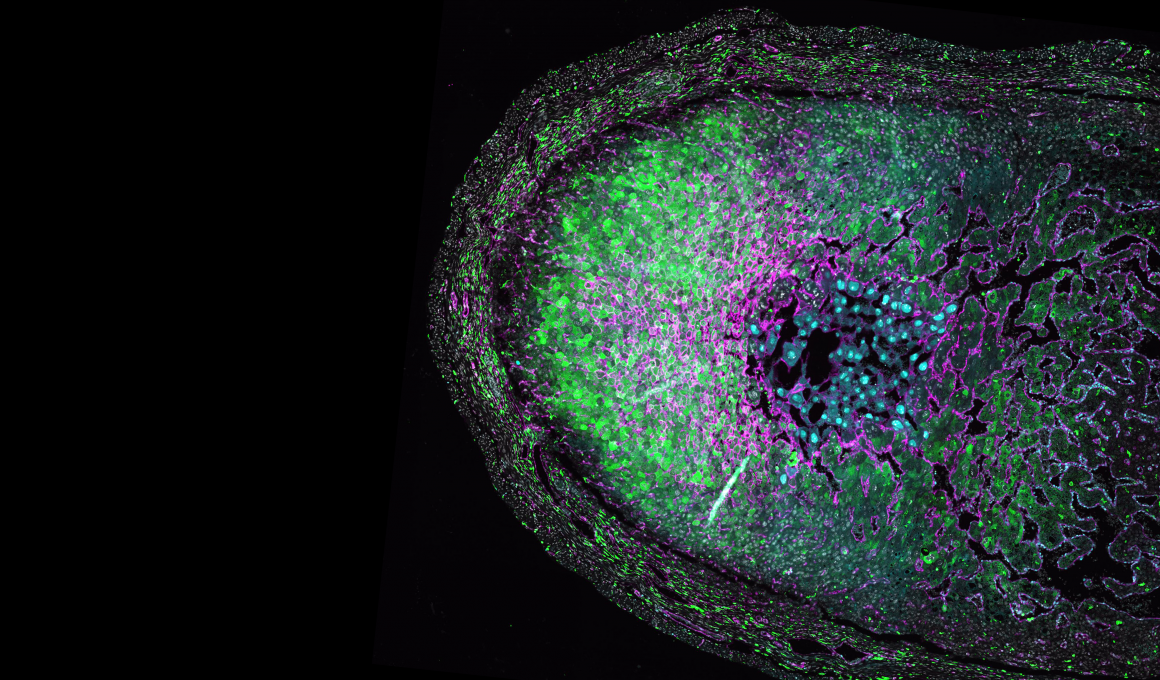
However, one place you have lymphoid tissue is deep within your nasal passages. Last year, in a groundbreaking study published in Nature, the Crotty Lab at LJI demonstrated the power of a simple nasal-swab procedure to collect immune cells and even measure SARS-CoV-2-specific immune cells from nasal lymphoid tissue in SARS-CoV-2-vaccinated or infected individuals.
So I thought: Maybe we can find lupus-driving immune cells in nasal lymphoid tissue of lupus patients. Not only is nasal swabbing a novel technique to sample immune cells in humans, but it allows for repeated sampling. With this technique, we can potentially monitor lymphoid tissues over time to track disease progression in patients, which is key for such a complex and dynamic disease like lupus.
Your project is ongoing, but can you share any of the results you’ve gathered so far?
A: Well, the big question at first was whether we could even get useful samples from this tissue, and the short answer to that question is: yes, we definitely can.
To date, we’ve enrolled 10 lupus patients with the help of our collaborating UC San Diego rheumatologists. Several of these participants have nearly completed a year of monthly sampling. The data we’ve collected from these nasal swabs show striking differences in the immune cell populations in lupus patients compared with swab samples from healthy controls.
Similarly, there are significant differences in immune cells collected from nasal swabs compared with immune cells isolated from the blood of lupus patients. Together, these data tell us that we are likely seeing lupus-specific immune cells in nasal swab samples. These cells are not found in the blood, so we’ve essentially identified multiple immune cell populations in nasal lymphoid tissue that have never before been studied in lupus.
Collecting longitudinal samples from the same lupus patients has also revealed some tantalizing bits of data. For example, we collected nasal swab samples and blood from a patient before and after they received a B cell-depleting therapeutic. We have been able to observe dramatic differences in B cells between the blood and nasal lymphoid tissue. And we were able to measure this effect over multiple time points post-treatment. Additional data from other donors show an increase in certain immune cell populations that seem to coincide temporally with disease flares, suggesting we might be capturing lupus-driving immune cells from our swab samples.
“We think this holds a lot of potential for utilizing nasal swabs as a means of monitoring responses to treatment or disease flares in lupus patients, in addition to furthering our fundamental understanding of how specific immune cell populations drive autoimmune disease.”
– LJI Instructor Carolyn Moderbacher, Ph.D.
Lupus is nearly 10 times more common in women than men. Could your research help us understand the reasons for this sex-based difference?
A: The sex bias in lupus is always in the back of my mind. A lot of autoimmune diseases are much more common in female patients, and we don’t know why. Genetics is likely a large component of the female bias in lupus. Genetics could be driving fundamental differences in immune cells in women—which could cause autoimmune disease under the right conditions.
My project aims to identify and understand lupus-specific immune cells in patients. If we can identify these disease-driving cells, then we can start to ask what genetic factors might be working together in those specific cells to make them more pathogenic than immune cells from people without autoimmune disease. This work could identify even more potential targets for new lupus therapeutics.